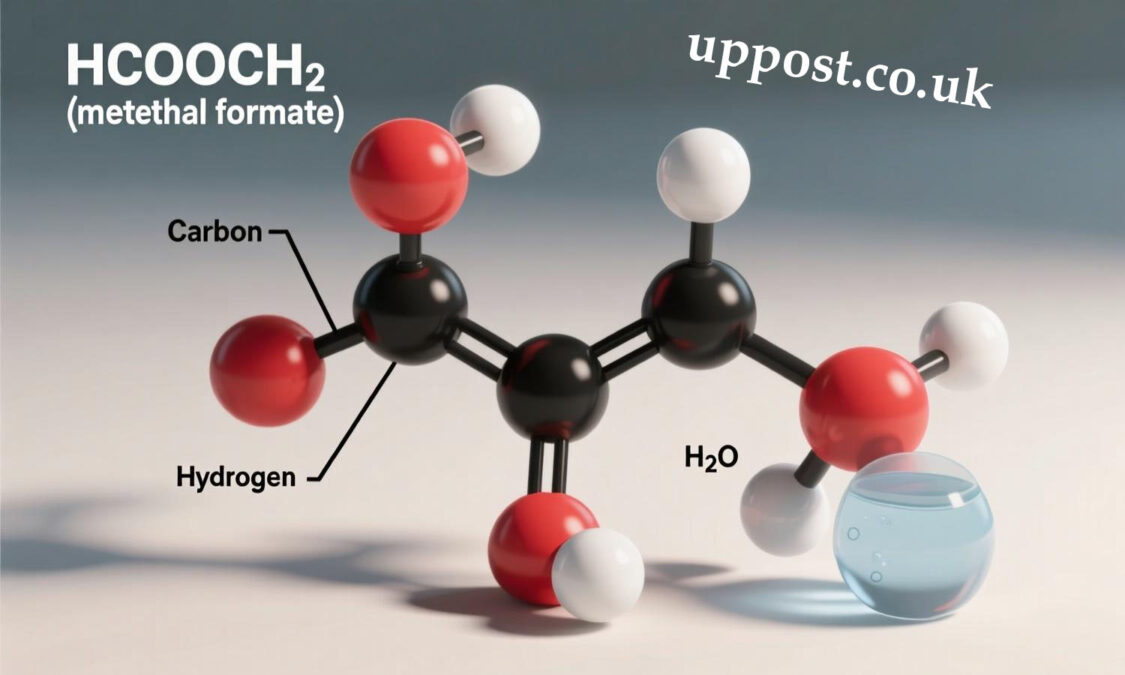
In the realm of organic chemistry, chemical compounds often present intriguing structures and diverse functionalities that impact industries ranging from pharmaceuticals to manufacturing. One such compound that has garnered interest in various scientific fields is hcooch ch2 h2o. This molecular formula represents a combination of functional groups that can be found in numerous chemical reactions and applications. In this article, we will delve into the structure, properties, uses, and significance of hcooch ch2 h2o, exploring its chemical makeup and relevance in multiple disciplines.
Chemical Composition and Structure of HCOOCH2H2O
The formula HCOOCH2H2O suggests a combination of ester, alcohol, and water functional groups. Breaking this down:
- HCOO refers to the formate group, which is derived from formic acid (HCOOH), a simple carboxylic acid.
- CH2 represents a methylene group, a carbon atom bonded to two hydrogen atoms.
- H2O denotes a water molecule, a universal solvent and reactant in many chemical processes.
Thus, HCOOCH2H2O could be interpreted as an ester (formate) bonded with a methylene group, which in turn is attached to a water molecule. This molecular arrangement might represent an intermediate or a byproduct of various organic reactions, especially those involving esterification or hydrolysis.
Applications of HCOOCH2H2O
The HCOOCH2H2O compound is not commonly encountered in isolation in everyday life but plays a crucial role in several industrial and research applications:
1. Solvent in Chemical Synthesis
In organic synthesis, water and formate esters are frequently used as solvents and reagents for various reactions. The presence of a water molecule (H2O) in the formula indicates that it could serve as an aqueous medium in many reactions, allowing for better solubility of organic compounds, reducing side reactions, and controlling reaction rates. This makes compounds like HCOOCH2H2O essential in controlled chemical environments.
2. Intermediate in Esterification Reactions
The HCOO group in HCOOCH2H2O is characteristic of ester bonds, which are vital in the esterification process. This reaction is key in producing various chemicals such as perfumes, plastics, and pharmaceutical agents. The formate ester group acts as a precursor or intermediate compound in these processes, making the understanding of HCOOCH2H2O valuable in chemical engineering.
3. Hydrolysis Reactions
The presence of water in the formula makes HCOOCH2H2O an excellent candidate for hydrolysis reactions. In hydrolysis, esters such as formates break down in the presence of water, producing alcohol and an acid. The reverse reaction, where water acts as a catalyst, is crucial in many biochemical and industrial processes, including the production of biofuels and detergents.
4. Formulation of Cosmetics and Pharmaceuticals
Ester-based compounds are prevalent in cosmetic and pharmaceutical formulations. They are used to create emulsions, as their ability to interact with both hydrophilic (water-loving) and lipophilic (fat-loving) substances allows for better product consistency. Thus, HCOOCH2H2O might be found as part of emulsifiers or stabilizers in these products.
Chemical Reactions Involving HCOOCH2H2O
Several chemical reactions involve compounds with similar functional groups to HCOOCH2H2O. Some of the key reactions include:
1. Esterification
Esterification is a reaction between an alcohol and a carboxylic acid (or its derivatives, like the formate group) to form an ester and water. If HCOOCH2H2O is part of such a reaction, the product would involve the exchange of the water molecule with an alcohol or other reactants, creating a new ester.
2. Hydrolysis
When exposed to water, formate esters like HCOOCH2H2O undergo hydrolysis, breaking down into their constituent components. In this case, HCOO would break down into formic acid (HCOOH), and CH2 could lead to the formation of methanol or other alcohols.
3. Condensation Reactions
In some cases, the combination of HCOO, CH2, and H2O could also participate in condensation reactions, where the removal of water facilitates the bonding of molecules to form larger, more complex compounds. These reactions are essential in the formation of polymers, resins, and other large molecular structures.
Importance of Understanding HCOOCH2H2O in Chemistry
The study of compounds like HCOOCH2H2O is important for several reasons:
- Innovation in Chemical Manufacturing: Understanding how molecules like HCOOCH2H2O interact in different conditions enables chemists to design better chemical processes, optimize reaction yields, and reduce waste.
- Environmental Impact: Esters and water play crucial roles in environmentally-friendly reactions, such as biodiesel production and green chemistry initiatives.
- Industrial Applications: From pharmaceuticals to polymers, the versatility of molecules containing ester and water functional groups means they can be applied across a wide range of industries.
Frequently Asked Questions (FAQs)
Q1: What is the chemical formula for formic acid, and how does it relate to HCOOCH2H2O?
The chemical formula for formic acid is HCOOH. It is a carboxylic acid from which the HCOO (formate) group is derived. hcooch ch2 h2o could be seen as an ester of formic acid that also includes a methylene group and a water molecule.
Q2: Can hcooch ch2 h2o be used in biological systems?
Yes, similar compounds are often involved in biochemical processes such as enzyme catalysis, metabolism, and the formation of bioactive molecules. The ester and water functionality makes it suitable for interactions with biological molecules.
Q3: How does hcooch ch2 h2o function in industrial applications?
HCOOCH2H2O can act as a solvent, reagent, or intermediate in various chemical reactions such as esterification, hydrolysis, and condensation. These reactions are integral to industries like pharmaceuticals, cosmetics, and manufacturing.
Q4: What are the potential risks of handling compounds like hcooch ch2 h2o?
As with many chemicals, handling should be done with care, especially in industrial settings. Compounds involving ester groups can be irritants or cause allergic reactions in some individuals. Always follow safety guidelines when handling chemicals.
Q5: Is hcooch ch2 h2o involved in environmental processes?
Yes, compounds like hcooch ch2 h2o are involved in eco-friendly reactions such as biodiesel production and the breakdown of organic matter. Their role in green chemistry is significant in reducing environmental pollutants.
Conclusion
The compound hcooch ch2 h2o may seem like a complex formula at first glance, but understanding its structure and reactions reveals its essential role in chemical synthesis, industrial applications, and environmental processes. Its unique combination of ester, alcohol, and water functional groups provides versatility and importance in various scientific and industrial fields. As chemistry continues to evolve, compounds like hcooch ch2 h2o will likely remain pivotal in the development of new processes, materials, and technologies.